 |
|
|||||
|---|---|---|---|---|---|---|
 |
||||||
|
EXPERIENCE PRECLINA Specialists’ CRO in Autoimmune and Inflammatory Disorders Preclina, a leading non-clinical CRO specializing in autoimmune diseases, supports global competitiveness by swiftly delivering top-quality evaluation results for biotechs and pharmaceutical companies under new drug development, securing their position in the explosively expanding market of the autoimmune disease therapeutics. |
||||||
|
||||||
|
||||||
 |
Arthritis Research Global Leading Service for Rheumatoid Arthritis Efficacy Evaluation and MOA ResearchTo facilitate the development of new drugs for rheumatoid arthritis, a prototype of chronic inflammatory disease, our scientists with over 20 years of expertise optimized the most reliable disease models. Preclina’s precise and highly reproducible test results have been recognized across diverse clients.
|
|||||
|
Fibrosis Research Robust Efficacy Evaluation Services for Anti-Fibrosis Drug DevelopmentPreclina has established a strong efficacy evaluation system for the development of next-generation fibrosis therapeutics that enables overcoming of the limitations of Nintedanib in the treatment of pulmonary fibrosis. Discover Preclina’s potent fibrosis model portfolio!
|
 |
|||||
 |
Inflammatory Skin Disease Research Providing Discovery Service of Psoriasis and Atopic Dermatitis Therapies to Carry Forward the Global New Drug LegacyAmid the emergence of prominent targeted antibody therapies, against inflammatory skin disorders, we offer full range disease model services encompassing from screening to confirmation.
|
|||||
|
Neurological Disorders Research and Development aimed at Surpassing the limitations of Clinical Approach treatmentsTry to verify the therapeutic efficacy of candidate compounds developed to address the unmet needs in the treatment of multiple sclerosis, where brain damage continues to progress despite attempts to suppress relapsing disorders. Experience our internally established, highly reproducible efficacy pharmacology models to confirm their therapeutic efficacy
|
 |
|||||
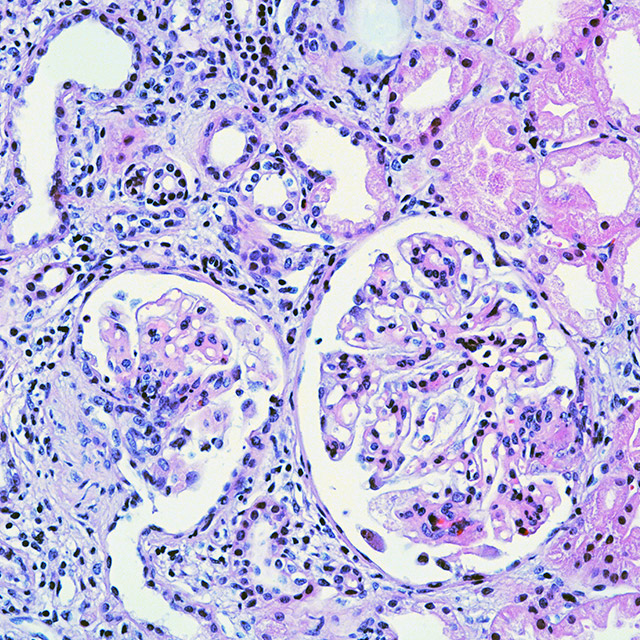 |
Collagen Vascular Disease Research Optimized Service for Drug Development Leader Groups Tailored to Disease MechanismsGiven the scarcity of clinically proven treatments, the field of lupus pharmacology demands focused drug development efforts. We are conducting efficacy assessments utilizing key lupus animal models to advance lupus therapeutic development.
|
|||||
|
Get in touch with us to find out more 
|
||||||
| Follow us | ||||||
|
|
||||||
|
HO : 719 & 1302, Teratower B, 167, Songpa-daero, Songpa-gu, Seoul 05855, Korea HO : 1st Bldg, 90 Chilgokjungang-daero 136-gil, Buk-gu, Daegu 41405, Korea T : 1566-0536| M : 010-2556-0536| E : service@preclina.com| unsubscribe |
||||||
