|
View this email online
|
 |
|
| Volume 8 |
 |
|

|
EXPERIENCE PRECLINA
Expert-validated Modular Platform for efficacy Evaluation Services
Count on Preclina’s modular platform for cutting-edge fibrosis drug development, from efficacy evaluation to advanced MOA research with our extensive immunological expertise and specialized fibrosis knowledge, we empower you to transform complex challenges into groundbreaking success.
|
SPOT-ON ACCURACY |

ROBUST CONSISTENCY |

BUDGET-SMART |

CUTTING-EDGE PLATFORM |
|
|
|
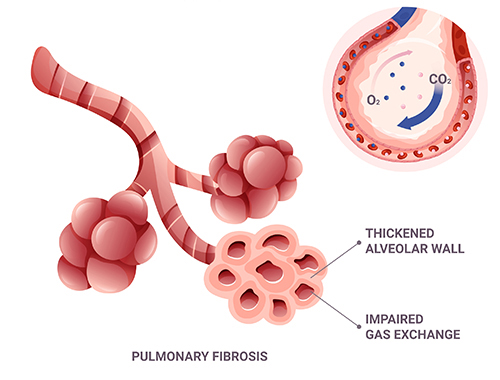
Efficacy Pharmacology for Pulmonary Fibrosis
Innovation & Lead
Advance pulmonary fibrosis therapeutics with our global standard models
evolutionize the therapeutic landscape for fibrotic lung diseases, including the formidable IPF.
Utilize our unparalleled efficacy pharmacology services to transcend the boundaries of current treatments and pioneer life-saving advancements.
|
|
|
 |
Master your research with global standard test models
|
|
 |
Expect unparalleled reliability that surpasses industry standards |
 |
Empower your decisions with cutting-edge read-outs
|
|
 |
Outpace competition with our swiftest results platform |
|
|
|
 |
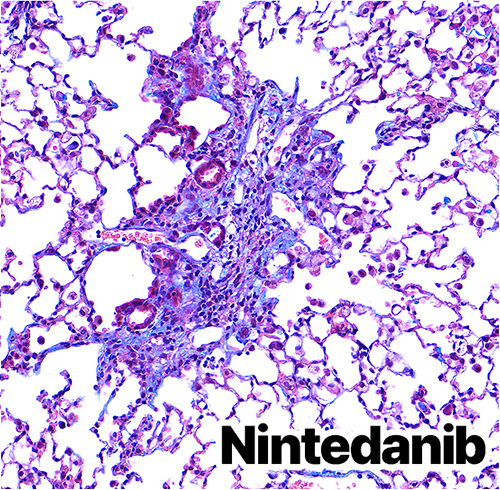
Bleomycin-induced Lung Fibrosis (BILF) Model
Breakthrough Targeted Therapeutics Surpassing Limitations of Nintedanib
As pioneers in the optimization of BILF models for developing pulmonary fibrosis therapeutics that surpass Nintedanib and Pirfenidone, Preclina is dedicated to exceeding our clients' diverse needs in drug development with unmatched service that perfectly balances cost-effectiveness and exceptional quality.
Mouse BILF Model |
|
Rat BILF Model |
| State-of-the-art standard models optimized for breakthrough drug development |
|
Fully compliant with different host requirements for IND approval |
|
|
|
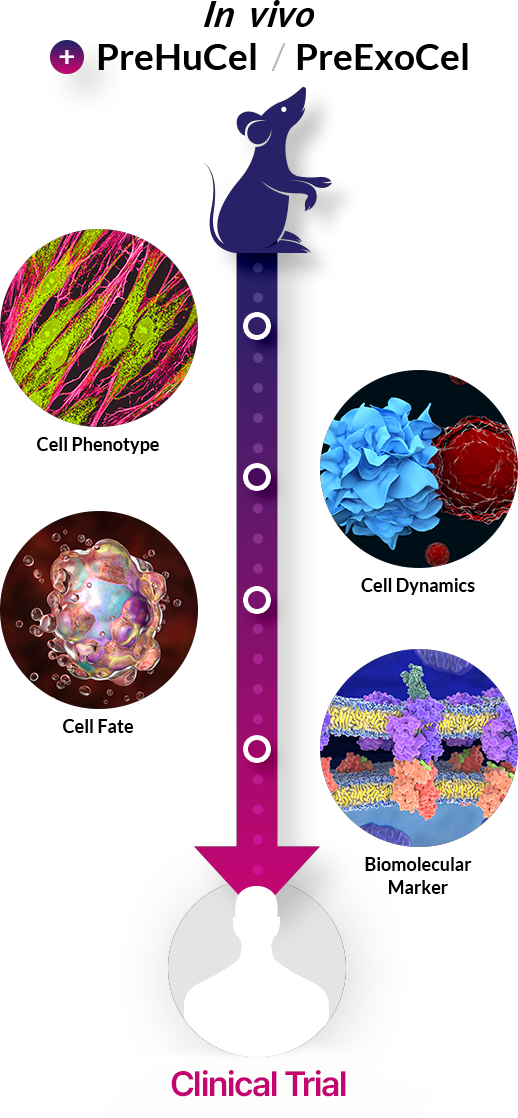
Patient-derived Cell-Based Assays
Comprehensive patient-derived primary cell banking for innovative drug pipelines
During technology transfer, requests for evidence of patient applicability often result in re-testing with patient-derived cells. Discover our cutting-edge solutions bridging the "death valley" between preclinical and clinical trials.
PreHuCel |
|
PreExoCel |
| Premier tool featuring customized technology for drug development |
|
Cell/tissue banking System across developmental stages of disease animal models |
|
 |
|
|
|

|
 |
Innovative Modular Platform
No shortcut? Don't detour!
Choose Pre-Xa services
Experience Preclina's modular platform service, ensuring reliable results from reproducible BILF studies with rapid outcome delivery. We will be your pathfinder through the labyrinth of drug development with our groundbreaking solutions.
PreBILFi Platform |
|
PreBILFi Premium Service |
| Revolutionizing every stage with precision-optimized workflow technologies |
|
A platform that integrates financial efficiency with pioneering technology |
|
|
|
Scleroderma Pharmacological Efficacy Assessment
An exceptional efficacy evaluation service with rare availability worldwide
Scleroderma can be partially delayed with treatments, but there are no proven anti-fibrotic drugs available. Preclina accelerates scleroderma drug development with a strategic system that utilizes an integrated portfolio of in vitro and in vivo fibrosis models along with a patient-derived biobank.
Bleomycin-Induced Skin Fibrosis Model |
|
Patient-derived Primary Cells |
| Global-standard model for scleroderma research |
|
Go/No-Go screening with pathologically relevant cells |
|
 |

|
|
|
|
Get in touch with us to find out more
about how we can support you and your research!

|
| Follow us |





|
|
HO : 719 & 1302, Teratower B, 167, Songpa-daero, Songpa-gu, Seoul 05855, Korea
HO : 1st Bldg, 90 Chilgokjungang-daero 136-gil, Buk-gu, Daegu 41405, Korea
T : 1566-0536| M : 010-2556-0536| E : service@preclina.com
|