Global Leading Autoimmune Specialists’ CRO
[OVERCOME CHALLENGES with PRECLINA]
[OVERCOME CHALLENGES with PRECLINA]
Preclina has launched the EXPERIENCE PRECLINA promotion to efficiently reduce the significant cost burdens for biotechs and pharmaceutical companies in evaluating the efficacy of novel drug candidates through highly optimized experiments.
Preclina, a leading non-clinical CRO specializing in autoimmune diseases, supports global competitiveness by swiftly delivering top-quality evaluation results for biotechs and pharmaceutical companies under new drug development, securing their position in the explosively expanding market of the autoimmune disease therapeutics.
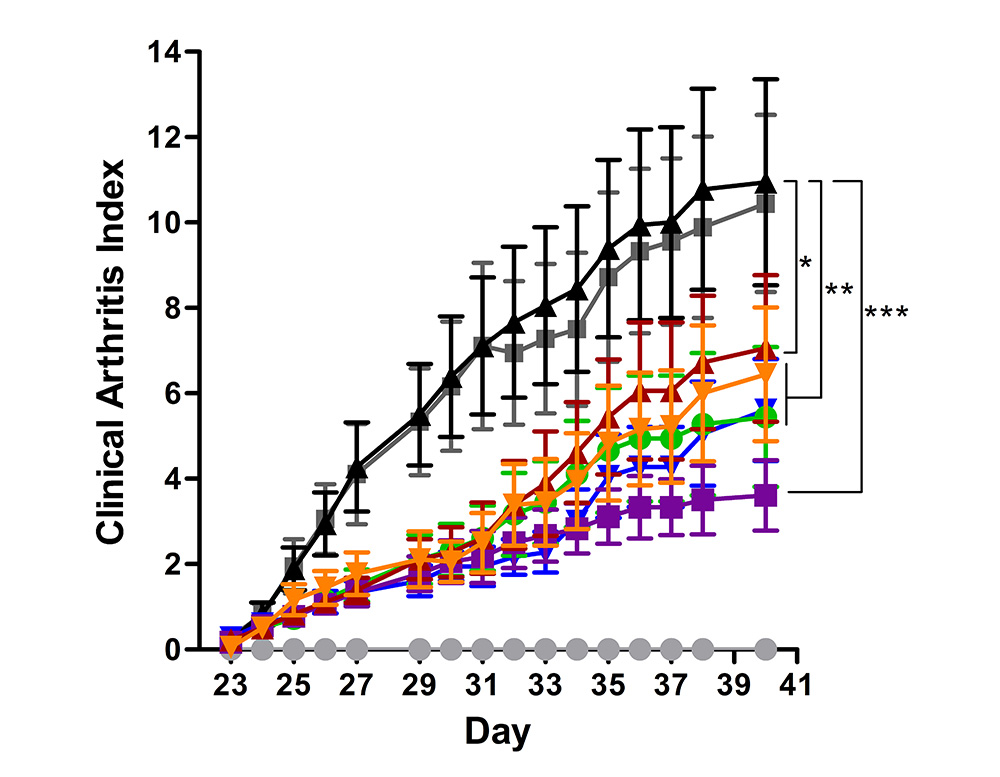
To facilitate the development of new drugs for rheumatoid arthritis, a prototype of chronic inflammatory disease, our scientists with over 20 years of expertise optimized the most reliable disease models. Preclina’s precise and highly reproducible test results have been recognized across diverse clients.
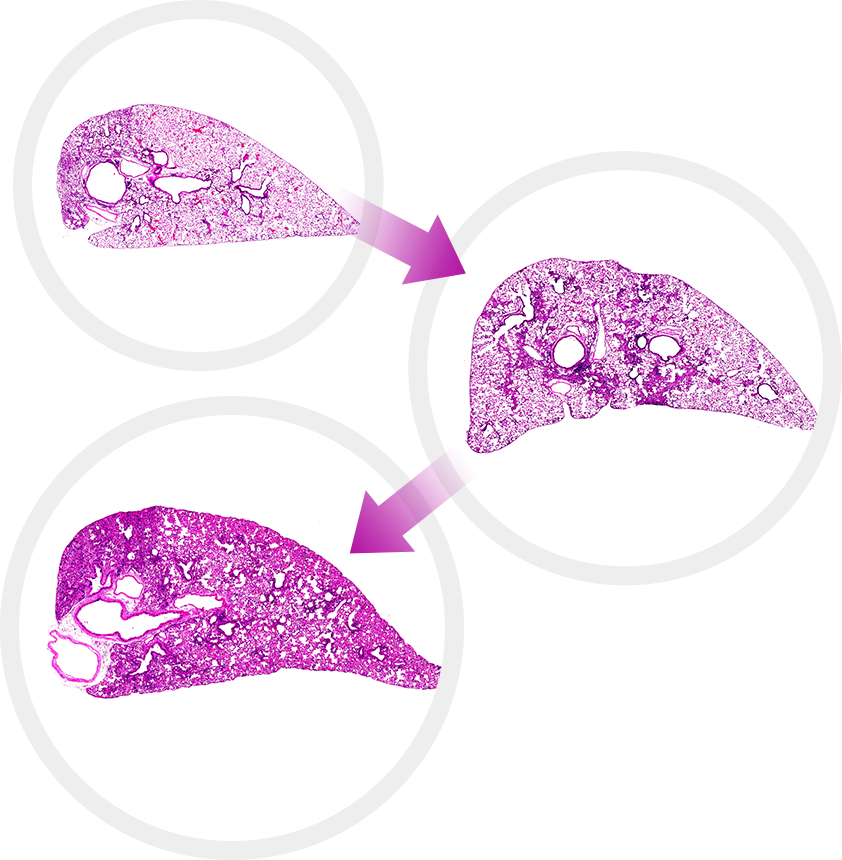
Preclina has established a strong efficacy evaluation system for the
development of next-generation fibrosis therapeutics that enables
overcoming of the limitations of Nintedanib in the treatment
of pulmonary fibrosis. Discover Preclina’s potent fibrosis model portfolio!
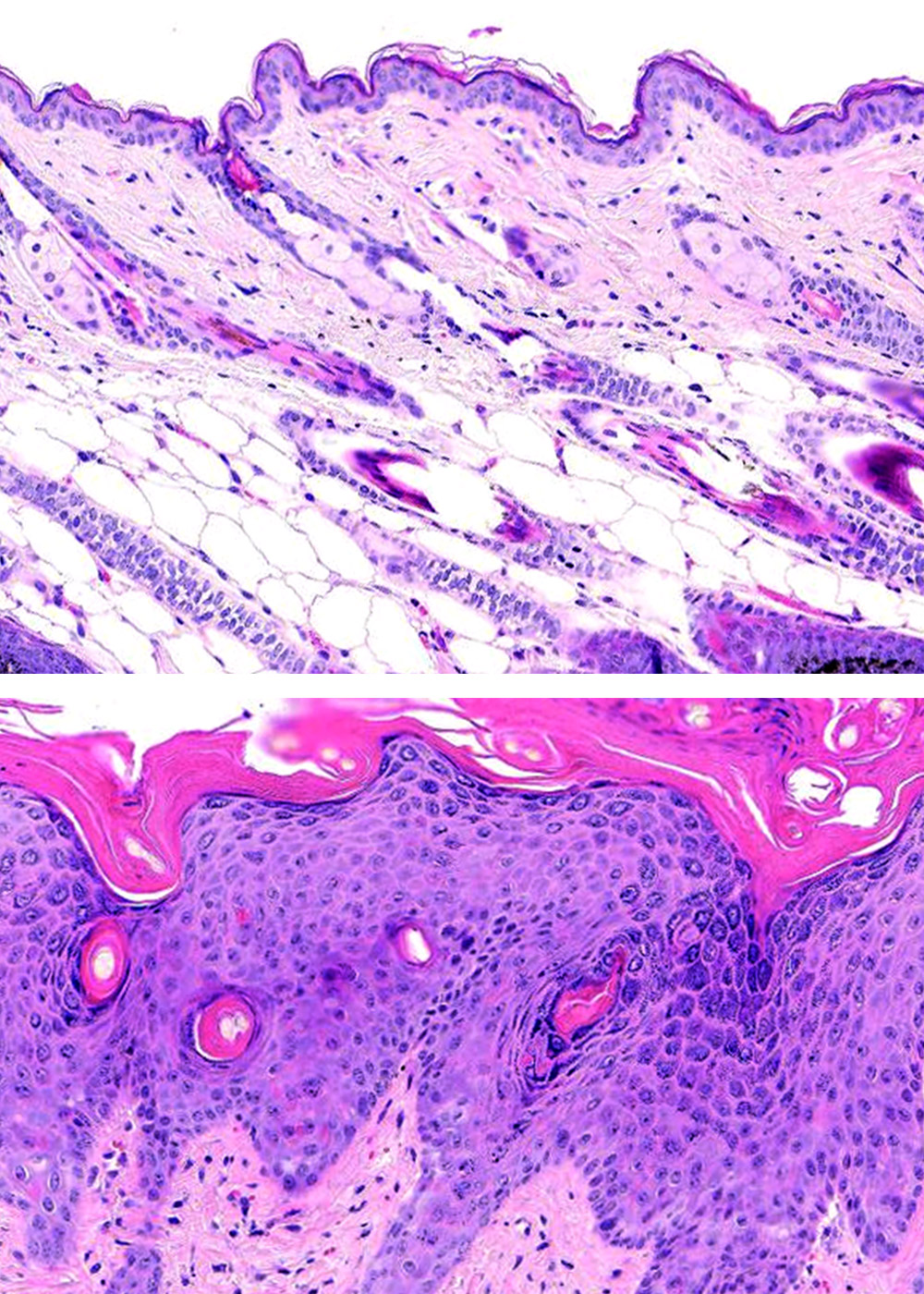
Amid the emergence of prominent targeted antibody therapies, against inflammatory skin disorders, we offer full range disease model services encompassing from screening to confirmation.
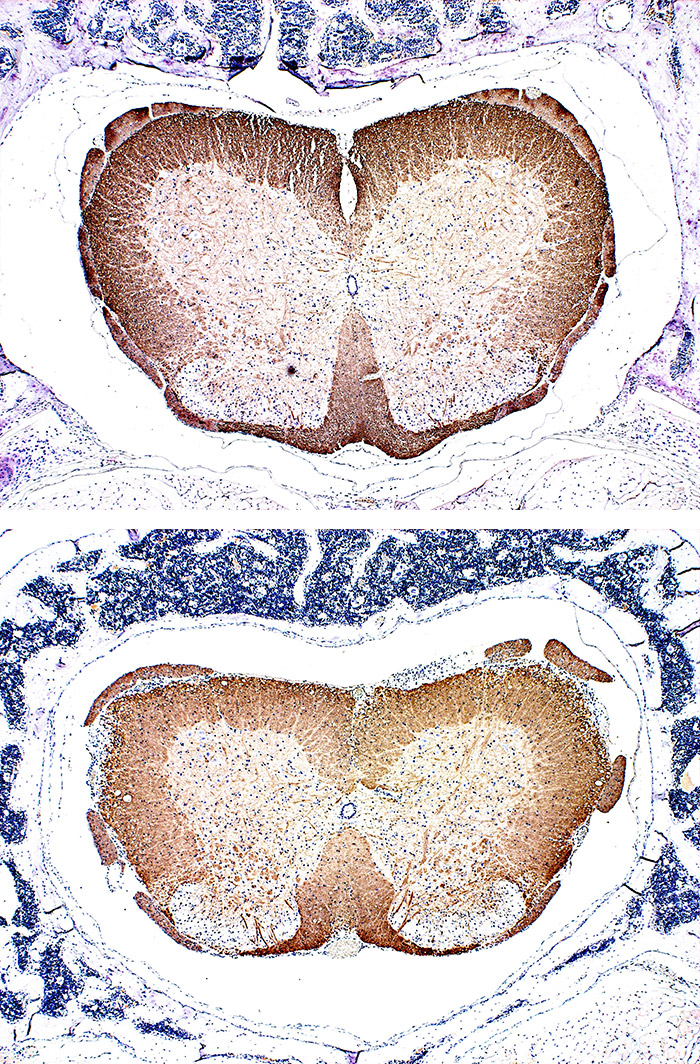
Try to verify the therapeutic efficacy of candidate compounds developed to address the unmet needs in the treatment of multiple sclerosis, where brain damage continues to progress despite attempts to suppress relapsing disorders. Experience our internally established, highly reproducible efficacy pharmacology models to confirm their therapeutic efficacy

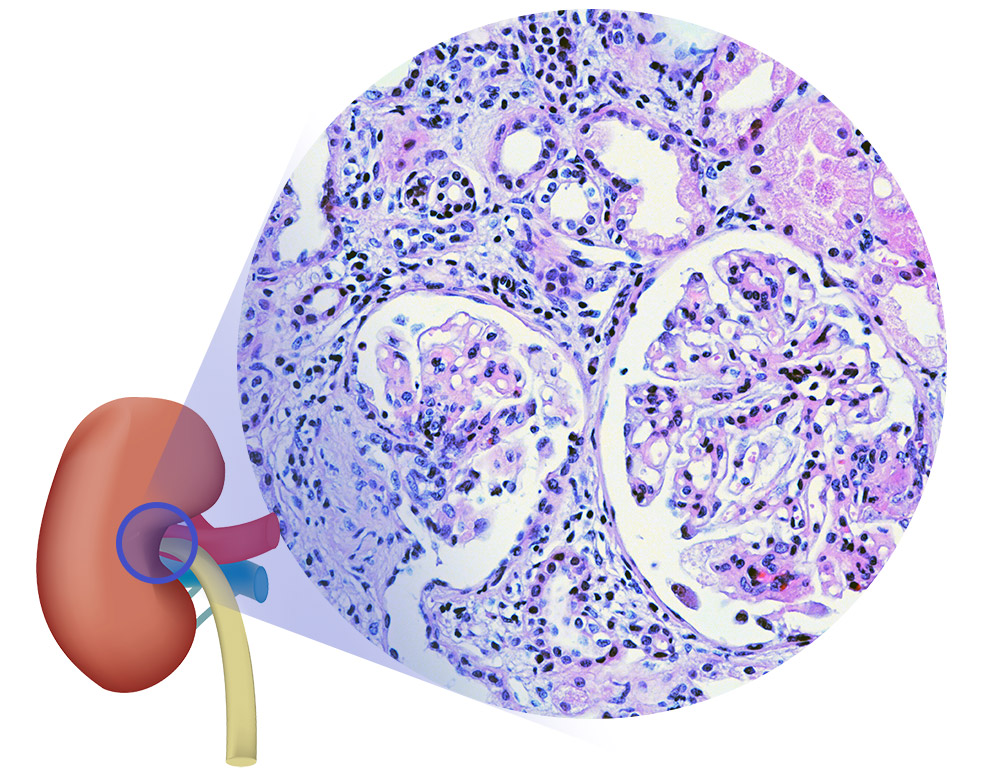
Given the scarcity of clinically proven treatments, the field of
lupus pharmacology demands focused drug development
efforts. We are conducting efficacy assessments utilizing key
lupus animal models to advance lupus therapeutic development.